Atom
- An atom is the basic unit of matter. It consists of a nucleus containing positively charged protons and uncharged neutrons, surrounded by negatively charged electrons that orbit the nucleus in shells.
Atomic Theory is given by John Dalton
Quark:
- The smallest part of the atom is called a quark.
Quark ‹ atom
Amedeo Avagadro
- Amedeo Avagadro’s Number = 6.022 x 1023. Avagadro’s Constant (NA) = 6.0222 x 1023 Mol-1
01 Mol of Carbon (C-12) in gram=6.022 x 1023
C-12 i.e 12 gram= 6.022 x 1023 or 0.12 gram= 6.022 x 1021 or 06 gram= ½. 6.022 x 1023
Nucleus, Electron, Proton, Neutron:
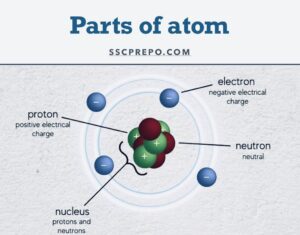
- Nucleus: Discovered by Rutherford
- Electron: Discovered by JJ Thomson and got the Nobel Prize in 1906. The charge on an electron is -1.6022 x 10-19 C and the Mass on an electron is 9.1 x10-31 kg. Note: JJ Thomson also discovered the Mass Spectrometer, which measures the Mass of the smallest Particle.
- Proton: Discovered by Rutherford and got the Nobel Prize in 1908. The charge on a Proton is +1.6022 x 10-19 C and the Mass on a proton is 1.67 x10-27 kg. Note: Goldstein discovered the Canal Ray/Anode Ray and later the same Canal Ray/Anode Ray known as Proton
A Canal Ray/Anode Ray is the ray of a proton and a Cathode Ray is the beam of an electron - Neutron: Discovered by Chadwick and got the Nobel Prize in 1935. The charge on a neutron is zero and the Mass on a neutron is 1.674 x10-27 kg.
- Positron (The Anti-Particle of Electron): Discovered by Anderson.
- Neutrino (The Chargeless and Massless Particle): Discovered by Pauli
- Atomic Number = Number of electrons/ or number of Protons
- Atomic Mass = Neutron + Proton [sum of both]
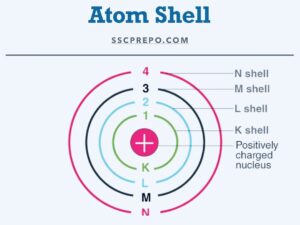
- Formula to find out the total number of electrons in the nth shell= 2 x n2
Atom
for example: K= 2 x 11 = 2, L= 2 x 22 = 8, M= 2 x 32 = 18, N= 2 x 42 = 32
where K, L M, N are the shells in which electrons revolve around the nucleus and s, p, d, f are the sub-shell of the K/L/M/N shell respectively. The number of electrons in the s, p, f, and d sub-shell are 2, 6, 10, and 14 respectively.
for example, Potassium (K)= 19
| Shell | K | L | M | N |
| Sub-shell | s | s,p | s,p,d | s,p,d,f |
| No of electrons | 2 | 2,6 | 2,6 | 1 |
No of electrons in the L shell are 08, and for stability 01 electron goes to the N orbit of sub-shell (according to the Octate Rule given by Kossel and Lewis)
Isotopes, Isobar, Isotones:
- Isotopes: Elements having the same number of protons (p) are called Isotopes.
for example, C-12, C-13, C-14 have same number of Protons = 6 - Isobars: Elements having the same number of atomic masses (p+n) are called Isobars.
for example, N-14 and C-14 have same Atomic Mass = 14 - Isotones: Elements having the same number of Neutrons (n) are called Isotones.
for example, C-13 and N-14 have same number of Neutrons = 7
| Elements | Isotopes
|
| (p)
|
| Isobars
|
| (p+n)
|
| Isotones
|
| (n)
|
| |
| C-12 | 6 | 12 | 6 | |||||||||
| C-13 | 6 | 13 | 7 | |||||||||
| C-14 | 6 | 14 | 8 | |||||||||
| N-14 | 7 | 14 | 7 |
- Here C-12 = atomic number of carbon is 12 and N-14= Nitrogen (atomic number 14)
- C-14 is a Radioactive element, used in the Carbon Dating Method to estimate the age of fossils.
- The uranium-lead method is used to estimate the life of Earth (life of Earth=3.5 billion years).
- Due to the Low “Half-life period” Isotopes are used for the detection or Treatment of diseases.
For example: a) Na-24: to detect Blood cancer
b) P-32: Blood cancer in WBCs (WBCs normal life=8k-10k, cancer WBCs life=20k-40k )
c) Co-60: used in general cancer treatment
d) I-131: For thyroid cancer
e) Radium-223: For Bone cancer
f) Arsenic-74: For Brain cancer
Proteium, Deutrium, Tritium
| 1H1 (Protium) |
1H2 (Deutrium) | 1H3 (Trituim) |
| 18Ar40 | 19K40 | 20Ca40 |
1H3 the lightest Radioactive element, discovered by Rutherford, 1H3 emits only β-Rays.
- In our earth’s atmosphere, the sequence of availability of elements
N2(78%)> O2(21%)>Argon(0.9%)> CO2(0.03%)
Among the inert gases, Argon is present maximum in the atmosphere. - Hydrogen is more than Helium in the universe.
Atomic Model:
- JJ Thomson Model: Also called Watermelon/Raisin/Plum-Pudding Model
- Neils Bhor Model: Also called the Planetary Model.
Heavy Water Vs Normal Water:
- Heavy Water(D2O): (Note: Not Hard water) Molecular Mass=20μ (2*2+16*1=20)
Discovered by Uray and Miler. Heavy water is used as a moderator in Nuclear reactions. The authorised centre for making heavy water in India is Bhabha Atomic Research Centre (Trombay-Mumbai-Maharastra). - Normal Water(H2O): Molecular Mass=18μ (1*2+16*1=18)